Abstract
Myeloablative conditioning or high-dose therapy followed by autologous hematopoietic cell transplantation (HDT-AHCT) is a standard of care (SOC) and potentially curative consolidation therapy for eligible patients diagnosed with aggressive lymphoma. Although effective in chemosensitive patients, HDT-AHCT is associated with severe regimen-related toxicities (SRRTs) that affect quality of life and may increase mortality risk (Olivieri et al., BBMT 2018). SRRTs occur due to the off-target cytotoxicity of HDT that involves damage to the microvasculature and the vascular stem cell niches of multiple organs. SRRTs predominantly affect organs with high cell turnover causing severe complications such as diffuse gastrointestinal (GI) mucositis (oral/GI SRRT) and delayed hematopoietic recovery. Rates and severity of toxicities increase with age, relevant to the demographics of the aging lymphoma population. Importantly, patients identify oral/GI SRRT as one of the most severe adverse effects experienced with HDT-AHCT (Stiff et al., BMT 2001). Notwithstanding, there are limited treatment strategies aimed at preventing SRRTs.
AB-205 is an experimental, engineered cell therapy that consists of allogeneic, human umbilical vein endothelial cells transduced with the pro-survival E4ORF1+ gene (E-CEL® cells). The proposed mechanism of action is paracrine in nature consisting of cell expression of reparative angiocrine factors which are thought to accelerate the repair injuries from off-target cytotoxicity of myeloablative conditioning. In a previously presented Phase 1/2 study [NCT03925935] in HCT-eligible patients with lymphoma, AB-205 was well tolerated and resulted in a 9% incidence of early oral/GI SRRT [defined as Grade ≥3 mucositis (M), nausea (N), vomiting (V) or diarrhea (D) in systemic lymphoma subjects conditioned with BEAM and BEAM-alternates (Phase 1/2 data is locked and analysis is in progress). This oral/GI SRRT incidence compared highly favorably to an incidence rate of 41% resulting from a contemporary, retrospective observational study conducted at two phase 1/2 sites (done concurrently during the conduct of the phase 1/2 study).
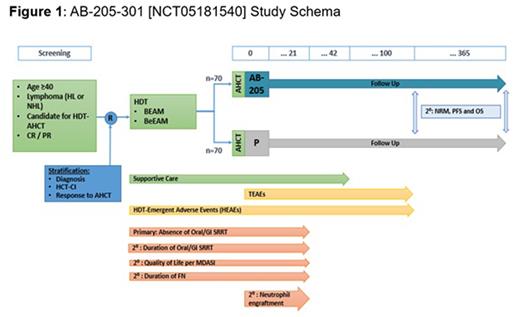
Accordingly, a phase 3, randomized, double-blind, placebo-controlled multi-center study (E-CELERATE) has been initiated to further investigate the efficacy and safety of AB-205 in reducing (preventing) severe toxicities and complications related to off-target cytotoxicity of myeloablative HDT. Approximately 140 patients will be enrolled across 25 sites in the United States.
Key inclusion criteria: Age ≥ 40; diagnosis of Hodgkin or non-Hodgkin lymphoma who are candidates for HDT-AHCT with one of the following myeloablative conditioning regimens: carmustine, etoposide, cytarabine, melphalan (BEAM); bendamustine, etoposide, cytarabine, melphalan (BeEAM); achieved complete response (CR) or partial response (PR) prior to planned HDT; ECOG ≤ 2; serum bilirubin ≤ 2 mg/dL; AST, ALT, and alkaline phosphatase < 3 × ULN.
Key exclusion criteria: Prior HCT; primary CNS lymphoma; systemic lymphoma with secondary CNS involvement at time of relapse prior to planned HDT-AHCT; active malignancy other than the one for which the subject is undergoing HDT-AHCT; subjects with a known history of HIV.
Endpoints: The primary endpoint is complete response defined as the absence of oral/GI SRRT (NCI CTCAE Grade ≥ 3 M, N, V, or D from the time of HDT through Day +21 post-HCT). Secondary endpoints include the duration of oral/GI SRRT; patient-reported symptom burden per MD Anderson Symptom Inventory; duration of febrile neutropenia; time to neutrophil engraftment; incidence of Grade ≥ 3 non-hematologic treatment emergent AEs; absence of SRRT; and survival endpoints: non-relapse mortality, progression free survival and overall survival.
Eligible patients will be randomized 1:1 to receive SOC supportive care prophylaxis + AB-205 or placebo intravenously (IV) given on Day 0 within 4 hours of AHCT. 20×106 cells/kg AB-205 IV or an analogous volume of placebo IV will be administered. Randomization will be stratified by lymphoma type (HL/NHL), hematopoietic cell transplantation-comorbidity index, disease status prior to HDT (CR/PR). Daily assessments of oral/GI SRRT will occur from start of HDT to Day +21 post AHCT (Figure 1).
Current status: Active recruitment and enrollment ongoing [NCT05181540].
Disclosures
Scordo:McKinsey & Company: Consultancy; i3Health (CME): Honoraria; Amgen, Inc.: Research Funding; Medscape, LCC (CME): Honoraria; Kite - A Gilead Company: Other: Ad-hoc advisory board (past); Omeros Corporation: Consultancy, Research Funding; Angiocrine Bioscience, Inc.: Consultancy, Research Funding. Shouse:Kite Pharma: Speakers Bureau; Beigene Inc USA: Honoraria. Gauthier:Juno Therapeutics - A BMS Company: Research Funding; Multerra Bio: Consultancy; Sobi: Research Funding; Legend Biotech: Consultancy; Kite Pharma: Consultancy; Celgene (A BMS Company): Research Funding. Dholaria:BMS: Research Funding; Vanderbilt University Medical Center: Current Employment; Wugen: Research Funding; MJH Biosciences: Honoraria; Molecular Templates: Research Funding; Arivan: Consultancy; Gamida Cell: Consultancy; BEAM Therapeutics: Consultancy; Janssen: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; Poseida: Research Funding; Angiocrine: Research Funding; Orca Bio: Research Funding; MEI Pharma: Research Funding; Jazz Pharmaceuticals: Consultancy. Rowley:ReAlta Life Sciences: Consultancy; SIRPant Immunotherapeutics: Consultancy. Pantin:Orca Bio: Research Funding; Cardinal Health: Honoraria; Omeros Corporation: Consultancy, Speakers Bureau; NKARTA: Consultancy. Defilipp:Incyte: Research Funding; Regimmune: Research Funding; Incyte: Consultancy; MorphoSys: Consultancy; Taiho Oncology: Research Funding; Syndax Pharmaceuticals: Consultancy; Kadmon: Consultancy; Omeros: Consultancy. Finnegan:Angiocrine Bioscience, Inc.: Current Employment.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal